kottke.org posts about periodic table
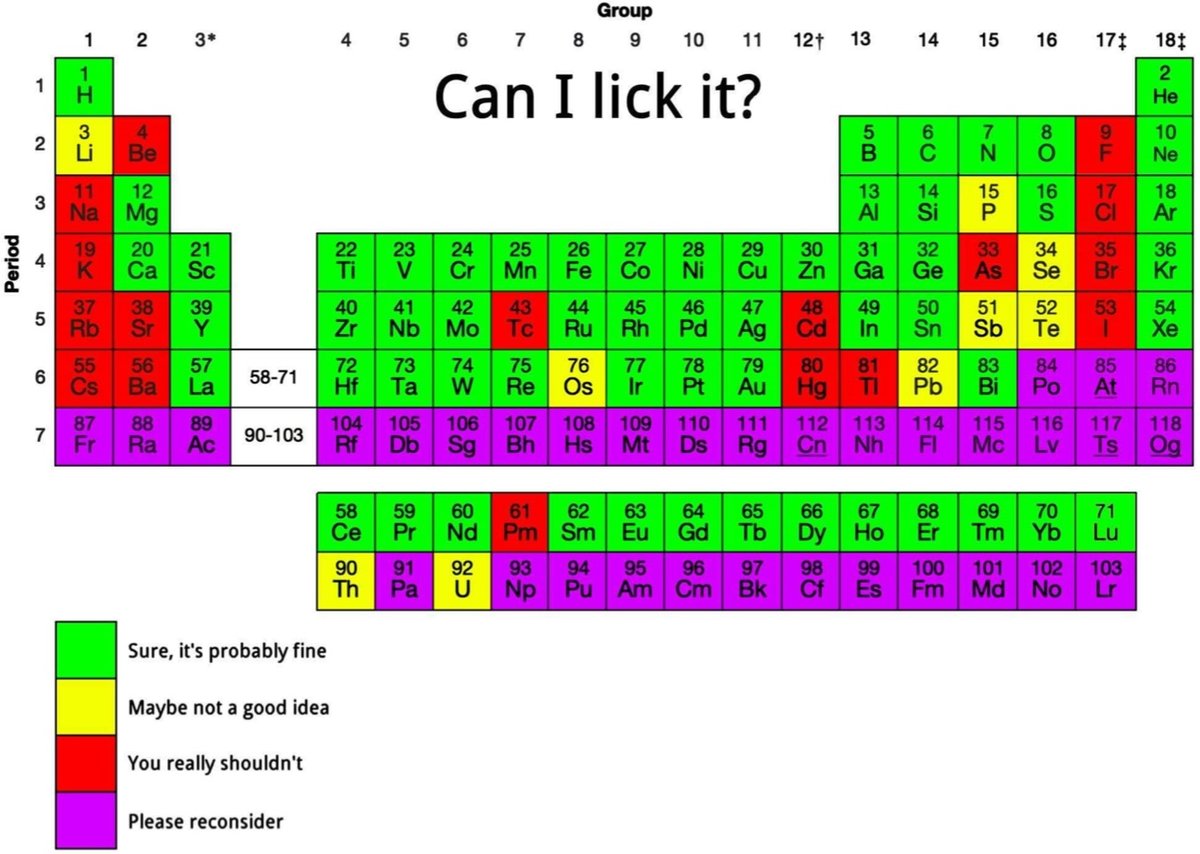
Is the periodic table yummy? Well, it depends on the element. But if you’ve ever wondered if a little taste of xenon or iridium would do you any harm, this periodic table is for you.
See also What If You Swallowed All Elements of the Periodic Table?. (via @anewplacetohide)
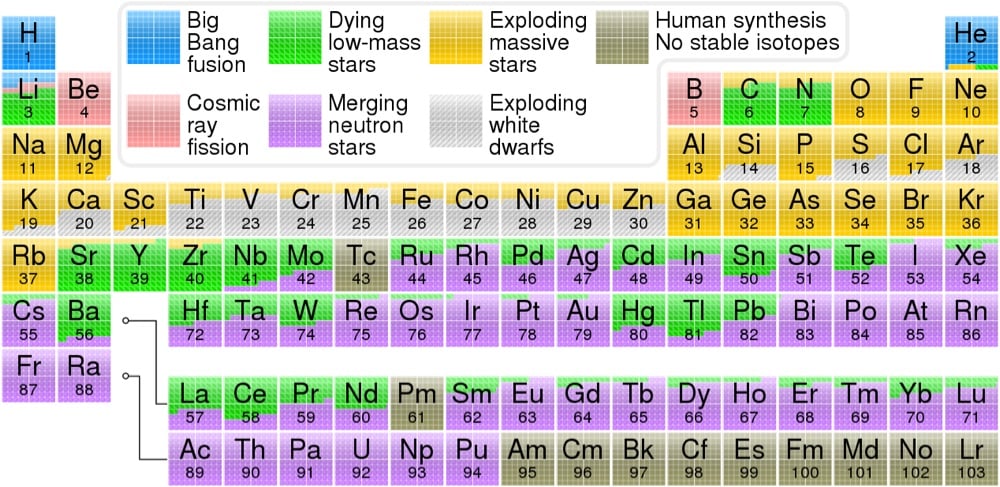
From Wikipedia contributor Cmglee and Astronomy Picture of the Day, a color-coded periodic table that displays which cosmic events — the Big Bang, exploding stars, merging neutron stars, etc. — was responsible for creating each element, according to our present understanding of the universe.
The hydrogen in your body, present in every molecule of water, came from the Big Bang. There are no other appreciable sources of hydrogen in the universe. The carbon in your body was made by nuclear fusion in the interior of stars, as was the oxygen. Much of the iron in your body was made during supernovas of stars that occurred long ago and far away. The gold in your jewelry was likely made from neutron stars during collisions that may have been visible as short-duration gamma-ray bursts or gravitational wave events.
The data for the table came from OSU’s Jennifer Johnson, who quotes Carl Sagan:
The nitrogen in our DNA, the calcium in our teeth, the iron in our blood, the carbon in our apple pies were made in the interiors of collapsing stars. We are made of starstuff.
(thx, caroline)
Bloomberg Businessweek dedicated their entire Sept 2, 2019 issue to the periodic table (it’s 150 years old this year) and the elements it contains. From the introductory essay:
Over the past century and a half, but particularly since World War II, scientists and engineers have learned to treat the periodic table like a banquet table-a bountiful spread from which to pluck what they need. There’s scandium in bicycle frames, tin (stannous fluoride) in toothpaste, tungsten in catheters, and arsenic in some computer chips. We are well past the Stone Age, the Bronze Age, and the Iron Age, and into the Everything Age, because almost every entry on the periodic table is being put to some kind of use in today’s economy (excluding synthetic elements that are costly to make and highly radioactive, such as einsteinium).
Cellphones exemplify the complexification. The first ones in the 1980s “were the size of a shoebox and consisted of 25 to 30 elements,” Larry Meinert, U.S. Geological Survey deputy associate director for energy and minerals, said in 2017. “Today, they fit in your pocket or on your wrist and are made from about 75 different elements, almost three-quarters of the periodic table.” That may include tantalum from Rwanda, potassium from Belarus, silver from Mexico, tin from Myanmar, carbon from India, and germanium from China.
Scrolling down on the main story page will take you on a modern-day tour of the periodic table from the lightest elements (hydrogen, helium, lithium) to the heavier ones (uranium, polonium) to some fake ones (adamantium, unobtanium, feminum).
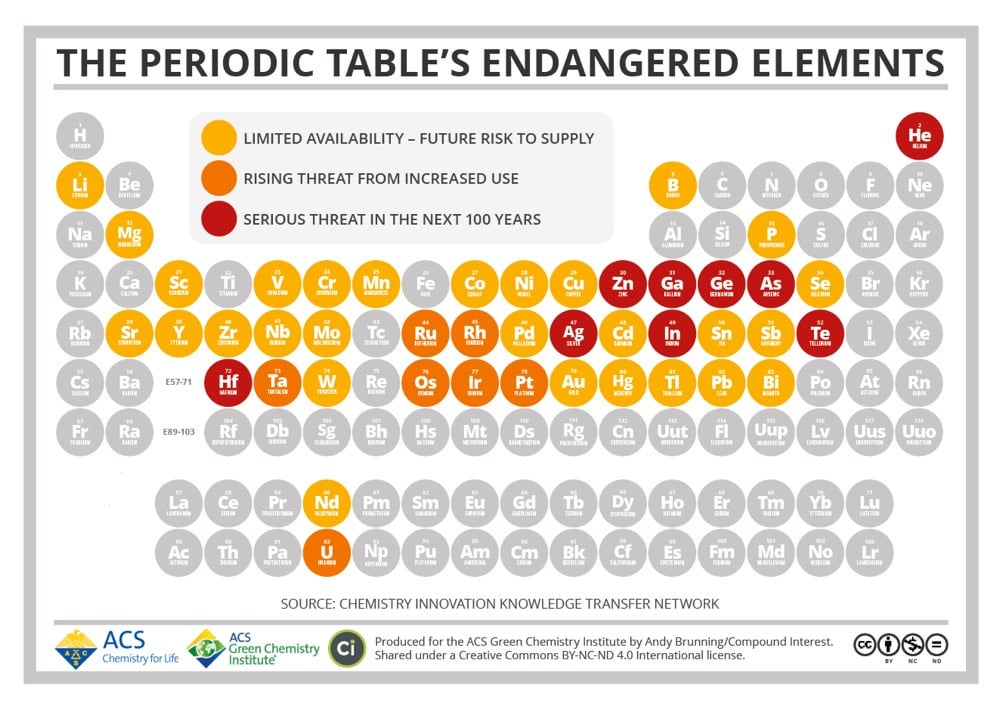
Until recently, humanity has treated the Earth as an infinite resource. As the Earth’s population has exploded over the past century however, we’ve learned in many different ways that that’s untrue. We’ve overfished the ocean, pumped too much carbon into the atmosphere and oceans, driven thousands of species into extinction, and terraformed much of the planet’s land. This periodic table produced by the American Chemical Society shows that there are also 44 chemical elements that will face supply limitations in the coming decades. Among those under a “serious threat in the next 100 years” are silver, helium, zinc, and gallium. Robert Silverberg wrote about The Death of Gallium back in 2008:
Gallium’s atomic number is 31. It’s a blue-white metal first discovered in 1831, and has certain unusual properties, like a very low melting point and an unwillingness to oxidize, that make it useful as a coating for optical mirrors, a liquid seal in strongly heated apparatus, and a substitute for mercury in ultraviolet lamps. It’s also quite important in making the liquid-crystal displays used in flat-screen television sets and computer monitors.
As it happens, we are building a lot of flat-screen TV sets and computer monitors these days. Gallium is thought to make up 0.0015 percent of the Earth’s crust and there are no concentrated supplies of it. We get it by extracting it from zinc or aluminum ore or by smelting the dust of furnace flues. Dr. Reller says that by 2017 or so there’ll be none left to use. Indium, another endangered element-number 49 in the periodic table-is similar to gallium in many ways, has many of the same uses (plus some others-it’s a gasoline additive, for example, and a component of the control rods used in nuclear reactors) and is being consumed much faster than we are finding it. Dr. Reller gives it about another decade. Hafnium, element 72, is in only slightly better shape. There aren’t any hafnium mines around; it lurks hidden in minute quantities in minerals that contain zirconium, from which it is extracted by a complicated process that would take me three or four pages to explain. We use a lot of it in computer chips and, like indium, in the control rods of nuclear reactors, but the problem is that we don’t have a lot of it. Dr. Reller thinks it’ll be gone somewhere around 2017. Even zinc, commonplace old zinc that is alloyed with copper to make brass, and which the United States used for ordinary one-cent coins when copper was in short supply in World War II, has a Reller extinction date of 2037. (How does a novel called The Death of Brass grab you?)
Zinc was never rare. We mine millions of tons a year of it. But the supply is finite and the demand is infinite, and that’s bad news. Even copper, as I noted above, is deemed to be at risk. We humans move to and fro upon the earth, gobbling up everything in sight, and some things aren’t replaceable.
As with many such predictions, the 2017 dates didn’t pan out, but the point that these resources are finite still holds. Eventually, we will run out.
IUPAC, the governing body for the official periodic table of elements, has announced the addition of four new elements to the table: ununtrium, ununpentium, ununseptium, and ununoctium. Those are working names…the teams that discovered each element has been invited to name them.
The proposed names and symbols will be checked by the Inorganic Chemistry Division of IUPAC for consistency, translatability into other languages, possible prior historic use for other cases, etc. New elements can be named after a mythological concept, a mineral, a place or country, a property or a scientist.
Ununoctium is so unstable that its half-life is 0.89 milliseconds and only three or four atoms of the substance have been produced in the past 10 years.
Google Research built an interactive periodic table of the elements where you can see the relative amounts of the elements as found in the human body, in the sea, and, most interestingly, by the number of mentions in books.
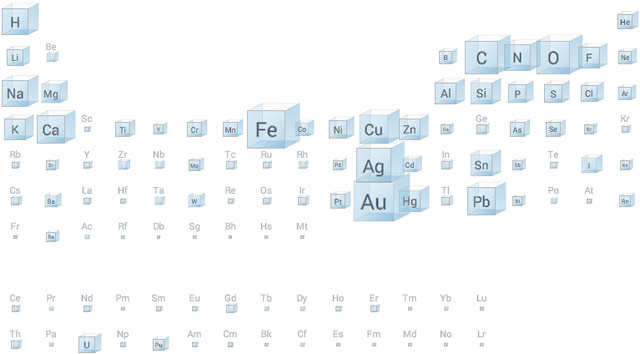
If you’ve ever wondered why the periodic table is shaped the way it is, click on “electrons” under “Shape” and pay attention to the number of electrons in the outer shells in each column of elements. Amazingly, when Dmitri Mendeleev and German chemist Julius Meyer published the first periodic tables in 1869/1870, the elements were organized only by atomic weights and chemical properties; they didn’t know what an electron was and certainly weren’t aware of quantum shells of electrons. (via @djacobs)
The elements located in the upper reaches of the periodic table are notable for their short half-lives, the amount of time during which half the mass of an element will decay into lighter elements (and other stuff). For instance, the longest lived isotope of fermium (#100) has a half-life of just over 100 days. More typical is bohrium (#107)…its half-life is only 61 seconds. The elements with the highest numbers have half-lives measured in milliseconds…the half-life of ununoctium (#118) is only 0.89 milliseconds.
So why do chemists and physicists keep looking for heavier and heavier elements if they are increasingly short-lived (and therefore not that useful)? Because they suspect some heavier elements will be relatively stable. Let’s take a journey to the picturesque island of stability.
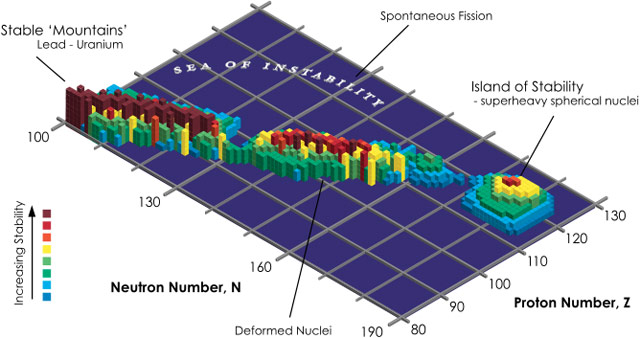
In nuclear physics, the island of stability is a set of as-yet undiscovered heavier isotopes of transuranium elements which are theorized to be much more stable than some of those closer in atomic number to uranium. Specifically, they are expected to have radioactive decay half-lives of minutes or days, with “some optimists” expecting half-lives of millions of years.
I would be fucking remiss in my duties here if I didn’t inform you of this bloody awesome periodic table of swearing, you bunch of stupid old wankers.
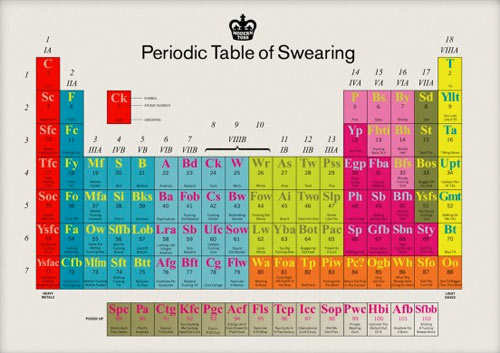
There’s goddamned prints available. (via clusterflock)
Sam Kean is blogging the periodic table of elements over at Slate.
Starting today, I’ll be posting on a different element each weekday (the blog will run through early August), starting with the racy history of an element we’ve known about for hundreds of years, antimony, and ending on an element we’ve only just discovered, the provisionally named ununseptium. I’ll be covering many topics-explaining how the table works, relaying stories both funny and tragic, and analyzing current events through the lens of the table and its elements. Above all, I hope to convey the unexpected joys of the most diverse and colorful tool in all of science.
If you like that, Kean has written a whole book on the topic.
A periodic table of awesomeness featuring Bacon as element #1, Laser as #21, and Black Holes as #82. I like bacon. Bacon is a close personal friend of mine. But can’t we keep this overexposed pork product out of it for once? (via rw)
The Periodic Table of Videos is a collection of videos about all the elements. All your favorites are there…Neon, Rubidium, Lead, Plutonium.
I did embarrassingly bad on this Elements of the Periodic Table quiz. I blanked after naming 17 elements in 2 minutes. Oh, and xylophone is not an element! My physics degree should be retroactively unawarded. (via mouser)
Periodic table of rejected elements, including Belgium, Antipathy, Visine, and Antigone. (via del.icio.us via kottke.org 8 years ago (it’s the time of year for recycled links, I guess))
An account of the discovery of Einsteinium and Fermium, elements 99 and 100 on the periodic table. They were generated by the detonation of Mike, the first hydrogen bomb to be tested.
Philip Stewart has constructed an alternate version of the periodic table of elements in the form of a “chemical galaxy”. “The intention is not to replace the familiar table, but to complement it and at the same time to stimulate the imagination and to evoke wonder at the order underlying the universe.”








Stay Connected