The island of stability
The elements located in the upper reaches of the periodic table are notable for their short half-lives, the amount of time during which half the mass of an element will decay into lighter elements (and other stuff). For instance, the longest lived isotope of fermium (#100) has a half-life of just over 100 days. More typical is bohrium (#107)…its half-life is only 61 seconds. The elements with the highest numbers have half-lives measured in milliseconds…the half-life of ununoctium (#118) is only 0.89 milliseconds.
So why do chemists and physicists keep looking for heavier and heavier elements if they are increasingly short-lived (and therefore not that useful)? Because they suspect some heavier elements will be relatively stable. Let’s take a journey to the picturesque island of stability.
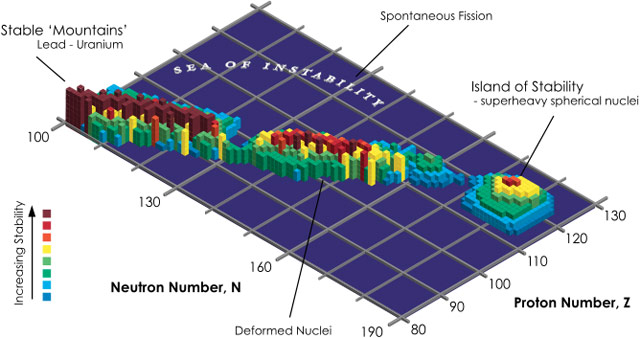
In nuclear physics, the island of stability is a set of as-yet undiscovered heavier isotopes of transuranium elements which are theorized to be much more stable than some of those closer in atomic number to uranium. Specifically, they are expected to have radioactive decay half-lives of minutes or days, with “some optimists” expecting half-lives of millions of years.




Stay Connected